Introduction: Standard treatments for older patients (pts) with acute myeloid leukemia (AML) can induce remission; however, responses are often short-lived and survival rates are poor upon relapse (Chen Y, et al. Medicine 2016;95:e4182). To date, no AML maintenance therapies have been approved by the US FDA, but a few have been used 'off-label'. Although some of these maintenance therapies, such as injectable azacitidine (AZA), improved disease-free survival in older pts with AML, overall survival (OS) benefits have been more difficult to achieve. Moreover, injectable therapies are associated with a higher administration burden and infusion reactions, and therefore may not be suitable for long-term maintenance therapy. The randomized, phase 3 QUAZAR AML-001 study (NCT01757535) of CC-486, a novel oral formulation of AZA, was the first maintenance study to demonstrate a significant and clinically meaningful improvement in OS (Wei AH, et al. Blood 2019;134:LBA-3). CC-486 was associated with a 9.9-month increase in OS (vs placebo) with a manageable safety profile and no compromise in health-related quality of life in pts with AML aged ≥ 55 years previously treated with intensive chemotherapy. Although previous research has examined patient preference for induction therapies, very little is known about preferences for AML maintenance therapies. We therefore used an online discrete choice experiment (DCE) survey to determine the relative importance that pts with AML place on key clinical benefits and risks, mode of administration, and out-of-pocket (OOP) costs.
Methods: From November 2019 to April 2020, pts aged ≥ 55 years from the USA, Canada, Germany, and Italy who had undergone treatment for AML were invited to participate in an online DCE survey. Attributes in the DCE survey included clinical benefits (time until relapse [6, 12, 24 months] and 2-year survival rate [30%, 50%, 60%]), adverse events (risk of mild-to-moderate stomach problems [40%, 60%, 80%] and risk of serious infection [10%, 20%, 40%]), mode of administration (once-daily oral tablet for 14 or 21 consecutive days/month, subcutaneous [SC] injection in a clinic/hospital for 7 consecutive days/month, or intravenous [IV] infusion in a clinic/hospital for 5 or 7 consecutive days/month), and OOP costs (USD 200, 400, 800). Patient preferences for attribute levels were analyzed using a multinomial logit model and expressed as marginal utilities and maximum acceptable decrease in 2-year survival rate.
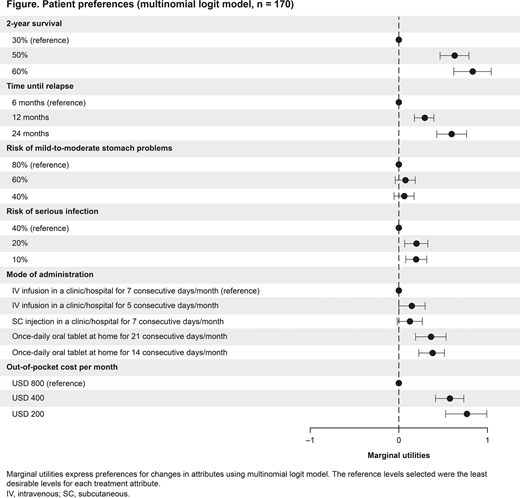
Results: In total, 170 pts completed DCE surveys (USA, n = 104; Canada, n = 6; Germany, n = 30; and Italy, n = 30). Mean age was 63.0 years, and 54% of pts were male. In all, 73% of pts had achieved remission at any time, 74% had not received prior stem cell transplant, and 79% had been diagnosed with AML in the last 6 months. Based on the DCE survey results, pts valued a 30% increase in the chance of 2-year survival (marginal utility for 60% = 0.84; 95% confidence interval [CI] 0.70-0.99) more than changes in any other attribute. This was followed by a USD 600 decrease in OOP costs (marginal utility for USD 200 = 0.77; 95% CI 0.66-0.89), 18-month increase in time until relapse (marginal utility for 24 months = 0.61, 95% CI 0.48-0.74), an oral tablet for 14 days/month instead of IV infusion 7 days/month (marginal utility = 0.38, 95% CI 0.23-0.54), and a 30% decrease in the risk of serious infection due to injection (marginal utility for 10% = 0.20; 95% CI 0.09-0.32). Risk of mild-to-moderate stomach problems was not important to pts (Figure). Pts preferred an oral tablet taken either 14 or 21 days/month over SC injection 7 days/month (both P = 0.002). In addition, pts were willing to accept a 16% and 14% decrease in the chance of 2-year survival to switch from IV infusion in a clinic/hospital for 7 consecutive days/month to an oral tablet for 14 days/month (95% CI 9.95-22.08) or 21 days/month (95% CI 7.44-20.50), respectively.
Conclusions: In this survey of pts with AML, the most important attribute during maintenance therapy was the probability of survival at 2 years. In addition, pts demonstrated a significant preference toward an oral tablet over IV infusions and SC injections in a clinic/hospital, and were willing to accept a significant decrease in treatment efficacy in favor of an oral mode of administration. This study provides valuable insights into patient preferences and may help inform decision-making for AML maintenance therapies.
Tervonen:Evidera: Current Employment, Current equity holder in publicly-traded company. Seo:Bristol Myers Squibb: Research Funding; Evidera: Current Employment. Nehme:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. La Torre:Bristol Myers Squibb: Current Employment. Prawitz:Bristol Myers Squibb: Research Funding; Evidera: Current Employment. Chen:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Beach:Bristol Myers Squibb: Current Employment. Wang:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal